The Benefits of Microporous Insulation in Fuel Cell Applications
Fuel Cell General Introduction:
What is a Fuel Cell?
A fuel cell is an alternative energy source that utilizes hydrogen to provide continuous energy without the use of an internal combustion operation. The reaction is “clean”, provides power comparable to or more efficient than current electric “grid”, diesel and gasoline generators, and other IC engines in automobiles. The reactions associated with the production of energy in fuel cells produce no harmful by-products and can be run (theoretically) without the use of diminishing fossil fuel reserves. Although still somewhat in the experimental stage, fuel cells can be used to power anything that requires electricity, including hand-held and commercial appliances, passenger vehicles, and large scale commercial and utility facilities where conventional energy sources are either not present or too expensive.
Fuel cell technology was originally “discovered” in the early 1800’s by an English scientist named Christian Friedrich Schoenbein, and though NASA and other national space programs have been utilizing fuel cell technology in space since the 1960’s, it is still a relatively new market for commercial and consumer use.
Due to the immense growth potential of the industry, numerous OEM’s for fuel cells have presented themselves and are already competing for shares in the inevitable market. As a result, there is tremendous pressure on all fronts to a) have working models in the field for testing and consumer purchase as quickly as possible, b) provide them in as cost effective a manner as possible to ensure survivability of individual fuel cell models, and c) ensure future growth by providing flexible variations that adapt themselves well to a variety of markets.
How does a Fuel Cell operate?
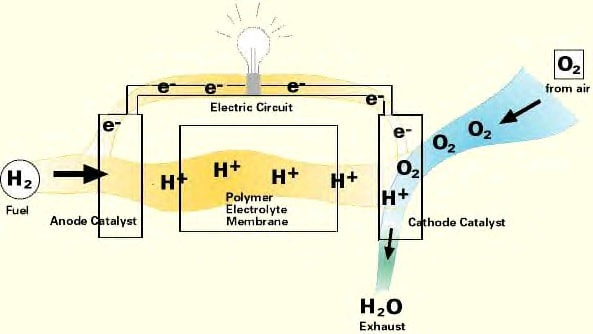
A fuel cell operates on the same principle as an “off the shelf” conventional battery, except that (as long as a fuel source is present) it does not run down or out of power over time. For most fuel cells, the ideal fuel source is hydrogen, either supplied directly or through conversion processes, and reacts with oxygen to produce by-products that are typically environmentally friendly (such as water and/or CO2, depending upon the fuel source and type of fuel cell). Depending upon the type of fuel cell, the hydrogen is then excited in some fashion as it passes through and from an anode, through an electrolyte membrane, and then to a cathode. This stream of excited hydrogen (i.e. hydrogen ions) is easy to convert to electric current and can be translated to “useable energy” for consumer applications.
In many instances, steam that results from a high temperature fuel cell will then recycle to a cogeneration mechanism like a micro turbine. This improves the overall efficiency of the fuel cell and ensures that all possible by-products are utilized for energy production.
The graphic below illustrates how the inner workings of an example fuel cell stack would operate.
Why do Fuel Cells matter?
Fuel Cells respond to the increasing demand for “Green” energy sources and potentially do not require immense reserves of ever-diminishing fossil fuels. They also do not produce the harmful by-products currently associated with internal combustion engines (since the by-products/waste-products of fuel cells are often easily useable by and naturally found in nature) and do not raise as many environmental concerns in their operation.
Fuel cells are relatively easy to maintain and service, can operate in very low temperature or very high temperature environments, and produce comparable energy output to other forms of energy production with improved overall efficiency. They are becoming increasingly more viable for a variety of industries and markets, and are expected to be a highly useful source for energy production in the years to come. Since fuel cells can theoretically power anything that requires electricity, they may be manufactured to be as small as a cigarette lighter (to power small, hand-held electronic devices), or as large as conventional power transformers. The example photographs below show some areas in which fuel cells are currently being employed.
How does Microporous insulation fit into the equation?
At some point during the fuel cell energy production process, either in converting hydrocarbons and natural gasses into pure hydrogen, or during the fuel cell’s internal processes themselves, there is substantial need for thermal insulation and heat containment. Microporous insulation is ideal in this instance as it can outperform other insulation systems by providing greater thermal performance with less and lighter material.
How does ThermoDyne fit into the equation?
In addition to being an OEM for microporous insulation, most fuel cells also require some form of complete systems engineering and fabrication. In many instances, this includes metal encapsulation and/or metal forming to coincide with the microporous parts being supplied, and ThermoDyne is ideally equipped to supply both. In addition, ThermoDyne is well positioned to supply custom or composite cut parts and specific shapes that may be necessary within a wide variety of fuel cell applications.
Types of Fuel Cells:
Alkaline Fuel Cells (AFC’s):
NASA and other national space programs have used AFC’s since the late 60’s and 70’s. Most of these types of cells use alkaline potassium hydroxide as the electrolyte in their reactions. They can also use a variety of fuel cell fuels (natural gas, hydrocarbon), but ammonia seems to be the fuel of choice. This, coupled with a reformer called an “ammonia cracker”, yields the pure hydrogen that the cell needs in order to operate.
Advantages: AFC’s can start and operate even at deep freeze temperatures (-40°Cent), making them ideal for aerospace and space applications, and operate at approximately 70% efficiency. They can also be shut down for rest or maintenance for long periods of time without the potential of water condensation and/or the costly and tedious process of removing or replacing component separators. They operate at higher voltage per cell (approx. 0.8v/cell) than PEM cells (approx. 0.6v/cell), and can operate on hydrogen derived from Ammonia rather than fossil or hydrocarbon fuels.
Microporous Benefits: Due to the relatively low operating temperature of AFC’s, the microporous opportunities tend to lie within their reformers or “ammonia crackers”. These reformers operate at 500 - 700°C (932 - 1292°F), and are ideal for microporous insulation.
Liquid Molten Carbonate (MCFC):
Liquid Molten Carbonate fuel cells are relatively high temperature fuel cells (1,200°F/650°C) that are able to operate without a noble metal catalyst (i.e. making them often very cost effective) and are able to use a number of different hydrogen sources as fuels. They are primarily targeted to be used in electrical and industrial utility applications, and have been successfully demonstrated in various markets around the world. They typically operate at approximately 60% efficiency, with an additional 25% added from cogeneration.
Advantages: Relatively high fuel-to-electricity efficiencies, operate on a wide variety of energy sources, proven testing and operation.
Microporous Benefits: Because molten carbonate fuel cells operate at temperatures above 1000°F, they are ideal candidates for microporous insulation. However, as they are not typically used in transportation or portable devices, the benefits of microporous insulation should be noted from a thermal efficiency point of view rather than from a space and weight savings angle.
Phosphoric Acid:
Phosphoric acid fuel cells are among the earliest players in the fuel cell market to produce working models. They operate at approximately 400 d F, and can be used to supply larger facilities such as airport terminals, hospitals, schools and utility power plants. The fuel cell stack operates at approximately 40% efficiency (i.e. 5% higher efficiency than electrical “grid” suppliers), with nearly 85% of resultant steam produced being recycled in cogeneration for additional energy output.
Advantages: Low operating temperature, already existing in the marketplace, fairly efficient with combined cogeneration.
Microporous Benefits: Due to the relatively low operating temperature of Phosphoric Acid fuel cells, there tend to be few microporous opportunities. However, as is the case with most fuel cells, there tends to be a high temperature reaction at some point in the overall process, and these would present themselves as possible opportunities for microporous insulation to be of use.
Proton Exchange Membrane (PEM’s):
These fuel cells operate with the use of a proton exchange membrane (typically coated on both sides with a precious metal such as platinum or gold) which allows hydrogen ions to pass through it. In conjunction with a standard anode and cathode on either side of the membrane, the metals on both sides act as catalysts for the reaction, and, as the hydrogen atoms pass through the membrane, releases ions which are utilized as electric current before passing to the cathode side of the fuel cell. Oxygen is fed into the cathode side of the fuel cell, where it then combines with the hydrogen, and forms water.
Advantages: Low operating temperature (80°C), quick start up when hydrogen is applied (making them ideal for automotive applications), very popular and widely accepted in both transportation and commercial markets.
Microporous Benefits: Microporous insulation is ideal, not as much directly in the PEM unit itself, but rather in the external or remote “reformers” which must be used to convert natural gas or hydrocarbons into the pure hydrogen necessary to run the PEM. These reformers typically operate at between 600-900°C (1050-1588°F), and since they are dependent upon maintaining that heat to break down the raw fuel, microporous is useful in containing heat and preventing heat loss (i.e. direct financial savings).
Solid Oxide Fuel Cells (SOFC’s):
SOFC’s are extremely high output fuel cells (some up to 220kW) that operate by using a hard ceramic core rather than a liquid electrolyte. They are being considered for both commercial/industrial and automotive/transportation applications, and come in a variety of shapes and sizes to meet customer requirements.
Advantages: Can use relatively “dirty” fuel sources, operates at approximately 85% efficiency, naturally uses carbon monoxide as secondary products to cogeneration and therefore naturally does not emit any harmful substances, does not need a reformer.
Microporous Benefits: Due to the high operating temperatures of these units, microporous insulation is ideal in containing and insulating the reactions. Whereas in many “combustion” type of environments, systems are passively cooled with water jackets or actively cooled with fans, it is still possible, with the use of microporous, to get the cold face temperature of many SOFC’s to “touchable” temperature.
For more information about ThermoDyne and/or any of its products or services, please contact us at [email protected]
Resources:
Photos and information resources courtesy of Fuel Cells 2000 and/or the US Dept of Energy (http://www.fuelcells.org)
Fuel Cell General Introduction:
What is a Fuel Cell?
A fuel cell is an alternative energy source that utilizes hydrogen to provide continuous energy without the use of an internal combustion operation. The reaction is “clean”, provides power comparable to or more efficient than current electric “grid”, diesel and gasoline generators, and other IC engines in automobiles. The reactions associated with the production of energy in fuel cells produce no harmful by-products and can be run (theoretically) without the use of diminishing fossil fuel reserves. Although still somewhat in the experimental stage, fuel cells can be used to power anything that requires electricity, including hand-held and commercial appliances, passenger vehicles, and large scale commercial and utility facilities where conventional energy sources are either not present or too expensive.
Fuel cell technology was originally “discovered” in the early 1800’s by an English scientist named Christian Friedrich Schoenbein, and though NASA and other national space programs have been utilizing fuel cell technology in space since the 1960’s, it is still a relatively new market for commercial and consumer use.
Due to the immense growth potential of the industry, numerous OEM’s for fuel cells have presented themselves and are already competing for shares in the inevitable market. As a result, there is tremendous pressure on all fronts to a) have working models in the field for testing and consumer purchase as quickly as possible, b) provide them in as cost effective a manner as possible to ensure survivability of individual fuel cell models, and c) ensure future growth by providing flexible variations that adapt themselves well to a variety of markets.
How does a Fuel Cell operate?
A fuel cell operates on the same principle as an “off the shelf” conventional battery, except that (as long as a fuel source is present) it does not run down or out of power over time. For most fuel cells, the ideal fuel source is hydrogen, either supplied directly or through conversion processes, and reacts with oxygen to produce by-products that are typically environmentally friendly (such as water and/or CO2, depending upon the fuel source and type of fuel cell). Depending upon the type of fuel cell, the hydrogen is then excited in some fashion as it passes through and from an anode, through an electrolyte membrane, and then to a cathode. This stream of excited hydrogen (i.e. hydrogen ions) is easy to convert to electric current and can be translated to “useable energy” for consumer applications.
In many instances, steam that results from a high temperature fuel cell will then recycle to a cogeneration mechanism like a micro turbine. This improves the overall efficiency of the fuel cell and ensures that all possible by-products are utilized for energy production.
The graphic below illustrates how the inner workings of an example fuel cell stack would operate.
Why do Fuel Cells matter?
Fuel Cells respond to the increasing demand for “Green” energy sources and potentially do not require immense reserves of ever-diminishing fossil fuels. They also do not produce the harmful by-products currently associated with internal combustion engines (since the by-products/waste-products of fuel cells are often easily useable by and naturally found in nature) and do not raise as many environmental concerns in their operation.
Fuel cells are relatively easy to maintain and service, can operate in very low temperature or very high temperature environments, and produce comparable energy output to other forms of energy production with improved overall efficiency. They are becoming increasingly more viable for a variety of industries and markets, and are expected to be a highly useful source for energy production in the years to come. Since fuel cells can theoretically power anything that requires electricity, they may be manufactured to be as small as a cigarette lighter (to power small, hand-held electronic devices), or as large as conventional power transformers. The example photographs below show some areas in which fuel cells are currently being employed.
How does Microporous insulation fit into the equation?
At some point during the fuel cell energy production process, either in converting hydrocarbons and natural gasses into pure hydrogen, or during the fuel cell’s internal processes themselves, there is substantial need for thermal insulation and heat containment. Microporous insulation is ideal in this instance as it can outperform other insulation systems by providing greater thermal performance with less and lighter material.
How does ThermoDyne fit into the equation?
In addition to being an OEM for microporous insulation, most fuel cells also require some form of complete systems engineering and fabrication. In many instances, this includes metal encapsulation and/or metal forming to coincide with the microporous parts being supplied, and ThermoDyne is ideally equipped to supply both. In addition, ThermoDyne is well positioned to supply custom or composite cut parts and specific shapes that may be necessary within a wide variety of fuel cell applications.
Types of Fuel Cells:
Alkaline Fuel Cells (AFC’s):
NASA and other national space programs have used AFC’s since the late 60’s and 70’s. Most of these types of cells use alkaline potassium hydroxide as the electrolyte in their reactions. They can also use a variety of fuel cell fuels (natural gas, hydrocarbon), but ammonia seems to be the fuel of choice. This, coupled with a reformer called an “ammonia cracker”, yields the pure hydrogen that the cell needs in order to operate.
Advantages: AFC’s can start and operate even at deep freeze temperatures (-40°Cent), making them ideal for aerospace and space applications, and operate at approximately 70% efficiency. They can also be shut down for rest or maintenance for long periods of time without the potential of water condensation and/or the costly and tedious process of removing or replacing component separators. They operate at higher voltage per cell (approx. 0.8v/cell) than PEM cells (approx. 0.6v/cell), and can operate on hydrogen derived from Ammonia rather than fossil or hydrocarbon fuels.
Microporous Benefits: Due to the relatively low operating temperature of AFC’s, the microporous opportunities tend to lie within their reformers or “ammonia crackers”. These reformers operate at 500 - 700°C (932 - 1292°F), and are ideal for microporous insulation.
Liquid Molten Carbonate (MCFC):
Liquid Molten Carbonate fuel cells are relatively high temperature fuel cells (1,200°F/650°C) that are able to operate without a noble metal catalyst (i.e. making them often very cost effective) and are able to use a number of different hydrogen sources as fuels. They are primarily targeted to be used in electrical and industrial utility applications, and have been successfully demonstrated in various markets around the world. They typically operate at approximately 60% efficiency, with an additional 25% added from cogeneration.
Advantages: Relatively high fuel-to-electricity efficiencies, operate on a wide variety of energy sources, proven testing and operation.
Microporous Benefits: Because molten carbonate fuel cells operate at temperatures above 1000°F, they are ideal candidates for microporous insulation. However, as they are not typically used in transportation or portable devices, the benefits of microporous insulation should be noted from a thermal efficiency point of view rather than from a space and weight savings angle.
Phosphoric Acid:
Phosphoric acid fuel cells are among the earliest players in the fuel cell market to produce working models. They operate at approximately 400 d F, and can be used to supply larger facilities such as airport terminals, hospitals, schools and utility power plants. The fuel cell stack operates at approximately 40% efficiency (i.e. 5% higher efficiency than electrical “grid” suppliers), with nearly 85% of resultant steam produced being recycled in cogeneration for additional energy output.
Advantages: Low operating temperature, already existing in the marketplace, fairly efficient with combined cogeneration.
Microporous Benefits: Due to the relatively low operating temperature of Phosphoric Acid fuel cells, there tend to be few microporous opportunities. However, as is the case with most fuel cells, there tends to be a high temperature reaction at some point in the overall process, and these would present themselves as possible opportunities for microporous insulation to be of use.
Proton Exchange Membrane (PEM’s):
These fuel cells operate with the use of a proton exchange membrane (typically coated on both sides with a precious metal such as platinum or gold) which allows hydrogen ions to pass through it. In conjunction with a standard anode and cathode on either side of the membrane, the metals on both sides act as catalysts for the reaction, and, as the hydrogen atoms pass through the membrane, releases ions which are utilized as electric current before passing to the cathode side of the fuel cell. Oxygen is fed into the cathode side of the fuel cell, where it then combines with the hydrogen, and forms water.
Advantages: Low operating temperature (80°C), quick start up when hydrogen is applied (making them ideal for automotive applications), very popular and widely accepted in both transportation and commercial markets.
Microporous Benefits: Microporous insulation is ideal, not as much directly in the PEM unit itself, but rather in the external or remote “reformers” which must be used to convert natural gas or hydrocarbons into the pure hydrogen necessary to run the PEM. These reformers typically operate at between 600-900°C (1050-1588°F), and since they are dependent upon maintaining that heat to break down the raw fuel, microporous is useful in containing heat and preventing heat loss (i.e. direct financial savings).
Solid Oxide Fuel Cells (SOFC’s):
SOFC’s are extremely high output fuel cells (some up to 220kW) that operate by using a hard ceramic core rather than a liquid electrolyte. They are being considered for both commercial/industrial and automotive/transportation applications, and come in a variety of shapes and sizes to meet customer requirements.
Advantages: Can use relatively “dirty” fuel sources, operates at approximately 85% efficiency, naturally uses carbon monoxide as secondary products to cogeneration and therefore naturally does not emit any harmful substances, does not need a reformer.
Microporous Benefits: Due to the high operating temperatures of these units, microporous insulation is ideal in containing and insulating the reactions. Whereas in many “combustion” type of environments, systems are passively cooled with water jackets or actively cooled with fans, it is still possible, with the use of microporous, to get the cold face temperature of many SOFC’s to “touchable” temperature.
For more information about ThermoDyne and/or any of its products or services, please contact us at [email protected]
Resources:
Photos and information resources courtesy of Fuel Cells 2000 and/or the US Dept of Energy (http://www.fuelcells.org)
